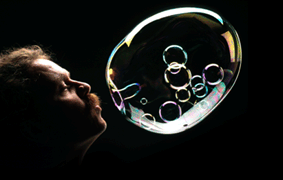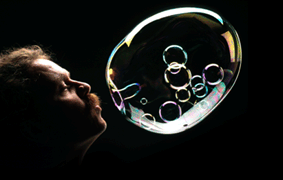SCHOOL SCIENCE PROJECT
<<Back to Kids page
Through my website I often receive letters from students who
are working on school projects. Some are very polite and some less
so. Often the students are asking that I simply hand over the information
that they’ve been assigned to discover. I do my best with the
email that I receive but, of course, with so many science projects
going on out there it would be impossible to engage in extensive
dialogue with everyone who writes. I often simply refer them to the
information that others and I have already written and put on their
websites.
But I had a very good exchange with a high school freshman who
used her own initiative as well as my contributions to put together
a nice presentation. I appreciated they way that she approached the
project and the way that she approached me and so I thought that
I would share that exchange with those interested.
Hi Tom Noddy,
My name is Elizabeth and I am doing a science project on bubbles. I was
wondering if you could send me some ideas on a neat demonstration that would
be easy enough to perform for my class, if you could, that would be great!
Thanks!
~ Elizabeth
Hello Elizabeth,
Hmmm ... maybe the others in your class think that bubbles would
pop if you tried to put something sharp inside of it. Try this at
home many times first:
1. Wet a table top (maybe a large cookie tray but a larger area
might be better. Wet it with bubble solution. Then, using a straw,
blow a bubble dome onto the wet surface. I mean, just blow a
bubble onto the wet surface and it will make a dome. Make sure that
the straw is wet deep into the bubble liquid (have some of the liquid
in a glass so that when you dip it it goes in deep and wets
the straw way up high)
2. Now put the straw inside of the bubble dome that’s on
the surface. Just push it in quickly ... if it’s wet it will
not break the bubble.
3. Now, while the end of the straw is in inside, blow another bubble.
A dome inside of a dome!
4. Pull the straw out quickly and then put it into
both domes and blow a third one inside.
So the straw doesn't break the bubble because it's wet and soapy
just like the bubble.
Now put something else inside of a bubble: a pin? no problem ...
if it's wet. A darning needle, a rose? It MUST be completely wet.
Dip the rose into the glass of soapy water and plunge it very quickly
into the bubble dome (if you go slowly, it won't go in, the bubble
will cling to the outside of the rose. Try this first with a smaller,
only partly open rose bud).
If you had a small pine tree and wet the needles you could decorate
the whole tree with bubbles till it looked like a Christmas tree
... as long as everything is wet and soapy.
Dip your finger into the soapy water ... put your finger inside.
What is a bubble? What force acts to keep all of those water and
soap molecules together into that network that we call a film or
a bubble? If you push a dry finger into that network you create a
gap ... the molecules are too far apart to hold together. If some
molecules let go of the net, then they all let go ... it's a network!
They all depend on each other. A dry finger (or rose, or needle)
will push those soap and water molecules away from each other but
... a wet finger is covered in the same kind of water and soap molecules
so they just become a part of the network. The bubble in now shaped
mostly like a dome and partly like a finger!
Do find out what that force is that keeps them together in the
first place ... it isn't gravity, that force would act to pull everything
down ... there is another force ... find out about that ... that's
part of the science in this project.
Good luck ... please let me know how this goes for you
Tom Noddy
Dear Tom Noddy,
THAT IS SO COOL! I am at a lack for words that can describe my
gratitude! Thank you so much for all the information and the BRILLIANT
ideas!! I am definitely looking forward to showing my class; wow!
Thanks also for responding to my e-mail so quickly, I need enough
time to perfect the "magic tricks!" Once again, I can not
thank you enough for all the help you have given me, (I will definitely
keep you updated on how it all works out, -time for me to go experiment!)
THANK YOU!
~ Elizabeth
P.S. I have been researching surface tension (of water), and have
figured out that the reason a water droplet stays together is there
is an inward pull from all the other molecules on the inside; since
there are no water molecules on the outside the droplet remains whole.
I am assuming that a bubble might be some sort of variation on this,
but I am still researching, THANK YOU ONCE AGAIN!!!
Elizabeth ,
I like that response. I get several letters asking for help with
school bubble projects and only sometimes do I hear back about them
at all and some of those are demands for more details. Yours was
charming.
Let me give you a couple of tips about the bubble solution:
On my website I recommend Mr Bubbles but the company that made
that has recently gone out of business. There seems to be some around
right now that is called Mr Bubbles but that I have not found to
be very good. Maybe the last of their bottles and labels are being
sold with an inferior mix.
So, for now, I would suggest that you use the liquid sold for use
in a toy called Gazillion Bubble Machine. They sell the containers
of liquid apart from the machine itself and it too is called Gazillion.
Several stores have it but if you don't see it then I know that Toys
R Us has it. It is unfortunately more expensive than Mr Bubbles or
Miracle bubbles but the bubbles, for your demonstration, will be
more stable longer.
Another tip Elizabeth. If you are having a problem when you're
doing this and the bubbles are popping it could be a few things:
dry air, dust in the air, old age, but really it is MUCH more likely
to be that the bubble touched something dry ... your finger? the
straw? (is it wet all the way up?), the edge of the cookie sheet?
... just wet everything and after a while wet them again. Don't
waste time scratching your head and blaming the conditions, just
wet everything again.
One other tip. The colors on a bubble are gorgeous (you already
knew that, I know). Those colors are best seen in the places where
the light is reflecting the brightest. Look closely. The reflection
of the light bulb itself or the reflection of the lampshade is the
prettiest. If you move the lamp closer, the reflection gets larger
so there is a larger pretty place.
Even better ... look at the colors against the backdrop of the
table or cookie sheet or white wall and now ... look at those same
colors with a black backdrop behind it. They REALLY stand out against
the black or dark colors.
I wonder how you could construct things so that your classmates
are seeing the bubbles against a darker not a lighter backdrop?
Could you cover the table or cookie sheet with something dark that
could handle the liquid? (if the blackness wasn't shiny that would
be best but sometimes you may need to compromise).
Again, good luck Elizabeth.
When you write back can you tell me what grade you're in.
Tom Noddy
Dear Tom Noddy,
All right!! So, the past few days I have been experimenting around with
bubbles! Although I didn't get the Gazillion bubbles mix, I have been successful. First
off, before I bought any bubble mix, I tried normal liquid dish soap. FLOP! It
didn't work, (but it did create quite a mess!) Next, I bought some kids bubble
bath and a "miracle bubbles" mix. The bubble bath was thicker than
the miracle bubbles so it was more successful. I was able to blow four giant
bubbles and stick what ever I liked into them. I even have a fake plastic rose
that I could use! I even brought put a paintbrush to show my younger brother
you could, "paint" on top of bubbles. He loved it, so did the rest
of my family. Thank you so much for the ideas! All of your tips were very, very
useful! I especially love the dark background idea! I painted a piece of cardboard
black and am using my dad's flashlight thing to illuminate it. Looks very cool!!
thank you so, so much!!
~ Elizabeth
P.S. I am in ninth grade!
Elizabeth ,
This is terrific. You're picking up on the tips that I've given
you but going your own way as well.
You are correct, of course, in thinking that soap bubbles are held
together and shaped by the same force that holds water drops together
-- surface tension. In the case of pure water the force is very great
(water has the highest surface tension of all of the common liquids).
In fact, it was long believed that the reason that you never see
a stable film of pure water is that the surface tension alone would
collapse the film from its own strength (more about this in a minute
...). So to make films we add soap to the water. The soap DECREASES
the surface tension of the water.
Try this:
Pour water into a glass all the way to the top.
Now, very slowly add more water.
Look ... you can add water above the top of the glass! Gravity
is trying to pull the water down but it is piling up above the
rim anyway. The force working against gravity in this case
is surface tension. The water molecules like each other more than
they like the air around them so they are clinging together and
they have enough strength to hold up a small pile of water.
That strength, the attraction of the water molecules for each other,
is what we call surface tension. We call it that because the effect
is so noticeable at the surface, where the liquid meets the air.
(Really, it's an electrical attraction ... the water molecules are
attracted to each other in a way that is similar to the way that
your socks are attracted to each other (and to other clothes) in
the laundry drier -- an electrical attraction.
Now try this:
Dip your finger into the liquid soap that you use to make the bubbles
with. Hold your finger above the glass of water so that a drop is
allowed to drip down onto the water.
That one drop will cause much of the water to run off of the bulging
top of the water. That's because soap decreases surface tension.
Elizabeth , I know that all of that talky science is a little less
fun than the bubble-blowing part but I can see that you're also interested
in the science and were interested before you and I started talking.
I think that you'll like this:
I said that it was long believed that the surface tension of pure
water would be enough to collapse a film of water before it could
stabilize but a few years ago an astronaut on the International Space
Station found out that that was not true. He planned to play with
soap bubbles in space (!) but the first thing he did was to demonstrate
that a pure water film wasn't possible ... then when he dipped a
wire loop into the water and took it out, he was shocked to find
a water film! It was forty times thicker than a soap/water film and
it was incredibly stable. One such film lasted for twelve hours.
So, it turns out that the reason that we've never seen a stable
pure water film is a combination of the high surface tension AND
the force of gravity. In space, where gravity is essentially zero,
*(see note below)* the film forms and stabilizes but it is so thick
that it would be too heavy to exist on earth. For more info about
this you can read the piece on my Science
page and from there you could link to the NASA website if you
got interested in learning more
But if you want to get back to blowing bubbles some more -- well,
I wouldn't blame you. They are beautiful, huh?
Tom Noddy
Dear Tom Noddy,
All right! Thank you once again for all the tips! I have completed my project
now, even the 5 page paper (urgh!) that took forever to write and my now wonderful presentation.
I will probably present to the class on Friday, and I will notify you on how
that goes. Since I have been practicing more and more, I have been able to get
bigger bubbles and once even fit five bubbles into a giant dome, which popped
quickly, but was accomplished! Also, your explanation on the water/detergent
thing was very useful, I was reminded of a time long ago when my brother was
into magic tricks that he made some pepper floating on water speed toward the
edges; (simply by dipping a finger dipped with detergent into the water). Now
I can show this trick to my classmates also, and have an explanation! Yes, you
are right about the talky part of science being a little more work, but I do
actually enjoy learning about what goes on around me, it just seems, well, fun
believe it or not!
~ Elizabeth
P.S. THAT SPACE STATION EXPERIMENT WAS SO INCREDIBLY COOL! I filled
up almost 2 pages talking about surface tension, and that was like
the icing on the cake!
P.P.S. It seems like lots of fun to experiment with bubbles in outer space,
doesn't it?
Elizabeth ,
Good luck on your presentation tomorrow. I'm glad that you've spent
so much time practicing with the bubbles ... there is really no substitute
for that. I've taught people how to do some of my tricks and others
have learned them on their own but simply spending time with the
bubbles will teach a lot about how this universe works.
You said in your last letter, speaking of the physics involved
in this bubble experiment, that you "actually enjoy learning
about what goes on around me". I'm delighted to hear that. It’s
probably too late to add anything to the presentation that you will
do tomorrow but, just for the sake of interest in the subject, I
will cite some more examples of the use that science has made of
knowledge obtained while looking into the common phenomena of soap
bubbles:
Soap bubbles have long been used as a model for studying the atomic
structure of metals and other crystals. Usually referred to as the
Bragg, Nye models where a layer of very small bubbles on the surface
of soapy water is seen to display grain boundaries, annealing, dislocation
and other properties associated with atomic interactions.
http://www.kstreetstudio.com/MatSci/Files/Meier-NEW-2000-3.pdf
Soap bubbles have been used as a model for the structure of the
universe itself. The latest maps of the sky suggest that the galaxies
and super galaxies are gathered in relatively thin boundaries and
separated by great "voids" just like bubbles in a froth.
(http://skyserver.sdss.org/edr/en/astro/structures/
structures.asp)
There are efforts underway right now to use a process of forming
and collapsing very small bubbles and through "sonoluminescence" (light
energy produced by generating sound!) producing heat that is greater
than that of the surface of the sun. Some hope that these teeny bubbles
will lead to an energy-efficient method of generating a larger
fusion reaction. If that were true it could eventually replace the
oil-based economy that we have today. http://ecow.engr.wisc.edu/cgi-bin/getbig/ema/519/crone/readingspd/bubble_education_paper_1_04preprintdraft.pdf
And recently bubbles were again used as a model by astrophysicists.
This time they found ways to apply the knowledge of a soap bubble's
surface tension to their study of "black holes". They are
surprised to find how well the model applies to these mysterious
powerful structures. http://www.physorg.com/news66408910.html
Elizabeth , it has been a pleasure to engage in this discussion
with you. I look forward to hearing about how the final presentation
goes in your classroom ... but the true reward is in the interest
that you have already taken in the subject.
I wish you the best.
Tom Noddy
Dear Tom Noddy,
Thank you soo much for all the help you have given me throughout
the project. First off, I'd like to say that my presentation went
off very well. The whole class was cheering for me as I blew the
bubble domes, and they all clapped when I was able to spear it and
blow more on the inside. At first, it was tough blowing the domes
because, (I’m thinking the air currents in the room caused
some problems) but because of all my practice, it turned out all
right. My science teacher was especially impressed and said he understands
everything that I explained to the class, which was a good thing!
Overall, my final grade in the project was an A! Once, again,
thank you ever so much for all the help that you had given me! I
don't think I could of gotten so far without all your expert advice!
THANKS AGAIN!
~ Elizabeth
Elizabeth ,
I'm very glad to hear that your hard work resulted in a good grade
as well as some fun and appreciation from your classmates. I'm glad
to have helped.
I've now copied our letters and am considering copying them onto
my website so that other students might benefit from a good example
of how the internet and email might be used to help a class project.
Also, some of your questions and my answers will be useful to students
or even just to people at home who want to play more successfully
with bubbles.
I would, of course, not include your email address or even your
name if I did this but I would use your letters and mine (after fixing
any misspellings). Please discuss this with your parents first and
then tell me what you and they think of the idea. I could communicate
the same information without using your words if they or you would
be more comfortable that way.
Meanwhile, good luck with your schooling and I can't help but encourage
you to continue to pursue an interest in science, you have a good
mind for it.
Tom Noddy
Dear Tom Noddy,
That would be fine, I talked to my parents and they don't mind at all if
you use the emails. I hope you don't have too many misspelling to fix! thanks
again,
~ Elizabeth
*(the term "zero gravity" is common but it is also incorrect.
It was a mistake for me to use this term. There is gravity
in space! It's what keeps the space station in orbit around
the planet, planets in orbit around the sun, solar systems within
galaxies and galaxies within galaxy clusters. We don't know of a
place that has "zero gravity" but when you are, as
the astronauts in orbit are, in freefall you experience
weightlessness and the soap film is reflecting that fact when it's
in orbit as well.)*
©Tom Noddy and Bubble Magic
P.O. Box 1576 • Santa Cruz, California • 95061 • USA • (831) 426-2230
Site design
by
Luckydog Arts
and Design©2006 |